2 Which of the Following Molecules Is the Strongest Acid
Phosphoric acid Ka 725 10³ larger Ka stronger acid Kw the ionization constant of water is. The substance CH3CH22NH is considered to be A.
Is hydrogen bonding strongest between molecules of H20 H2S H2Se or H2Te.

. B All Brønsted-Lowry bases contain a lone pair of electrons or a p bond. HClO4 HClO3 HClO2 HClO HF. Which of the following compounds is the strongest acid.
CH3COOH OR Ethanol. Correct option is C Among the given acids perchloric acid HClO 4 is the strongest acid. HF does not show any reducing character while HI is a strong reducing agent.
A hydrofluoric acid with Ka 35 10-4. The conjugate base C l O 4 has maximum delocalization due to which it is weakest base. For a generic weak base equilibrium you have Baq H 2Ol BH aq OH aq The base dissociation constant is defined as.
Which of the following compounds is both a. Explain your answer briefly in each case. Briefly explain your choices and show the reaction that occurs for BOTH of the molecules that you choose.
FCH2COOH is the strongest acid among the following. 1000 Acid precipitation has. Solution for Which of the following is the strongest acid.
10¹⁴ M² at 25C A pH of 6 is how many times more acidic than a pH of 9. HO CH Molecule 2. Which of the following statements about a Brønsted-Lowry base is true.
Which molecule is the a strongest base b weakest base. Hydrochloric acid is a strong acid. Explanation- An atom is considered to be a better acid if its conjugate base is stable.
Acetic acid is a weak acid. Briefly explain your answer in each case. What is the strongest monoprotic acid of the following set if all the acids are at 0100 M concentration.
There are only a few 7 strong acids so many people choose to memorize them. FCH2COOH ClCH2COOH BrCH2COOH ICH2COOH. ACH3NH2 bCH3PH2 cCH3OH dCH3SH.
Which is more soluble in water. Hydrogen selenide H2Se reacts with water according to the following equation. Among oxyacids of halogens the decreasing order of the acid strength is.
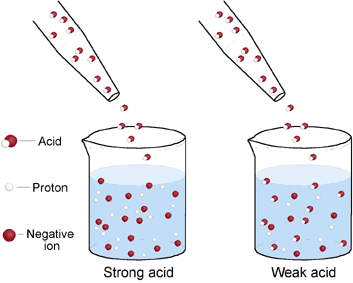
The following pictures represent aqueous solutions of three acids mathrmHAmathrmAmathrmX mathrmY text or mathrmZ with water molecules omitted for clarity. Since the electron withdrawing inductive effect -I effect of the halogens decreases in the order of FCl Br I therefore the acidic strength of the α- halo acids decreases in the same order. Which of the following molecules is the strongest acid.
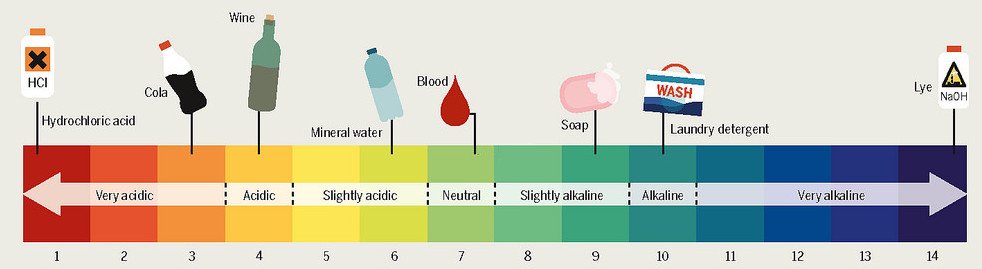
So H F is the weakest of the halogen acid and H I is the strongest acid. Chlorine has highest oxidation number 7. And when using the like dissolves like.
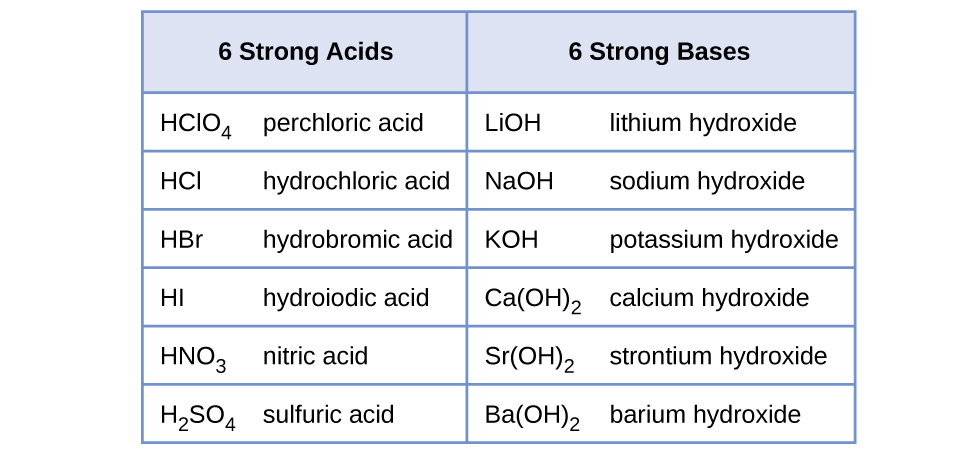
The strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid. Perchloric acid H C l O 4 or C l O 3 O H is the strongest acid. Provide a reason as to why.
Video Explanation Was this answer helpful. Para-Aminobenzoic acid PABA p-H2NC6H4COOH is used in some sunscreens and hair conditioning products. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid HF.
FCH2COOH is the strongest acid out of all. Bond is the strongest hence it requires maximum energy to dissociate. Correct answer is Molecule 3.
Hydrochloric acid denoted by the chemical formula HCl Hydrobromic acid denoted by the chemical formula HBr Hydroiodic acid or hydriodic acid denoted by the chemical formula HI Sulfuric acid denoted by. O dimethylamine CH3NHCH3 o ethanol CH3CH2OH o dimethyl ether CH3OCH3 O methane CH4 o propene propylene CH3CHCH2. HF CH3CH2CH2OH CH3CH2COOH which is the strongest to weakest acid.
Which of the following is the strongest acid. A The net charge may be zero positive or negative. Y HO CH Molecule 4.
It has maximum number of electronegative oxygen atoms 4 due to which more electrons will be pulled away from O-H. Which of the three is the strongest acid and which is the weakest. Which of the following compounds is the strongest acid.
Which molecule is the a strongest acid b weakest acid. Apr 08 2022 0755 AM Solutionpdf. ACH3OCH3 bCH3CH2OH cCH3CHO dCH3CO2H.
Which process defines how an ionic compound breaks apart into its constituent ions upon dissolution. C All Brønsted-Lowry bases contain a proton. Use patterns in.
View the full answer. The list of strong acids is provided below. A total of seven acids are widely regarded as strong acids in the field of chemistry.
H2Se H2O SeH H3O In three to five sentences identify the acid base conjugate acid and conjugate base in this reaction. AHF bH2O cNH3 dCH4. Which one of the following is a strong acid.
Which of the following compounds is the strongest acid. Mineral acids are stronger acidic nature than organic carboxylic acids. Which of the following molecules is the strongest acid.
HClO 4 HClO 3 HClO 2 HClO Hence option C is correct. Piperidine Kb 13 103 The strongest conjugate acid will correspond to the weakest base which in your case is the base that has the smallest base dissociation constant Kb. Hence we can understand that option d is the correct answer for the given question.
Which of the following molecules will be the second strongest acid. CH3CH2OH I think its acetic acid because when observing its structure it is more polar than ethanol. The answer to this question is.
A Molecule 4 O. D The net charge may be zero or positive. When the conjugate base is weak the acid is strong.
Carboxylic acids are strong. Ketamine Kb 30 107 E. This is because the conjugate base ClO 4 ion is the weakest base.
Access a diverse Question Bank and ask You Own Doubt Now. All the other acids are weak.

List Of Top 7 Strong Acids Turito Us Blog
Comments
Post a Comment